ukushima
[under construction]
C. Health Effects of Microwaves, including
Cell Phones and Cell Towers, and Electromagnetic Pollution – Non-Ionizing
Radiation
I. Lai Henry,
Comet Assay and Single-Strand and Double-Strand Breakage Caused by Non-Ionizing
Radiation.
http://www.envinfo.org/Lai_Henry.htm
electromagnetic
and radiofrequency radiation RF
http://microwavenews.com/news-tags/henry-lai
Lai, H. and N.P. Singh. (1995) "Acute Low-Intensity Microwave Exposure Increases DNA Single-Strand Breaks in Rat Brain Cells."Bioelectromagnetics 16: 207-210. https://www.ncbi.nlm.nih.gov/pubmed/7677797
Lai, Henry (2000a). "Biological Effects of Radiofrequency Radiation from Wireless Transmission Towers." In Levitt (2000) 65-74. See also http://cyrusfarivar.com/docs/WiFi%20Health/CELL%20TOWER.pdf
Lai, Henry. "Microwaves Break DNA in Brain; Cellular Phone Industry Skeptical." Microwave News 14, no. 6 (November/December 1994).
http://microwavenews.com/news/backissues/n-d94issue.pdf
Lai, H. and Singh, N.P. (1997a) : "Acute exposures to a 60 Hz magnetic field increases DNA strand breaks in rat brain cells". Bioelectromagnetics 18: 156-165.
https://ecfsapi.fcc.gov/file/10811208526661/ELF%20-%20Lai,%20Singh,%201997.pdf
https://ecfsapi.fcc.gov/file/10811208526661/ELF%20-%20Lai,%20Singh,%201997.pdf
p. 160
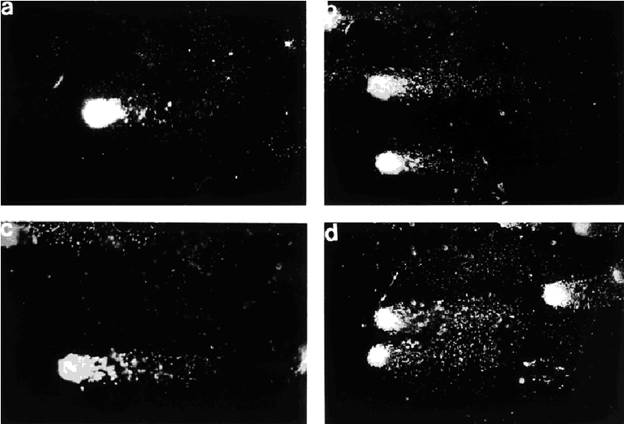
Fig. 6. Photographs of single-strand break DNA migration pattern of individual brain cells from
rats exposed to bucking condition (0.1 mT) (a) or magnetic fields of 0.1 mT (b), 0.25 mT (c),
and 0.5 mT (d). x 400.
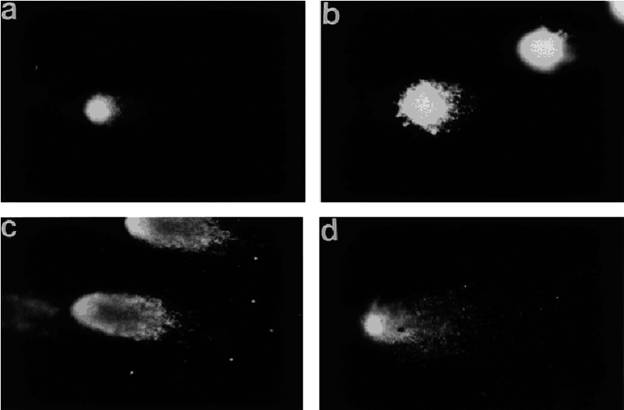
Fig. 12. 60 Hz Magnetic Field and DNA Strand Breaks
163
Fig. 12. Photographs of double-strand break DNA migration pattern of
individual brain cells
from rats exposed to bucking condition (0.1 mT)
(
a) or
magnetic fields of 0.1 mT (
b), 0.25 mT
(c), and 0.5 mT
(d).x 400.
Lai, H. and N.P. Singh. (1997b) "Melatonin and a Spin-Trap Compound Block Radiofrequency Electromagnetic Radiation-Induced DNA Strand Breaks in Rat Brain Cell." Bioelectromagnetics 18, no. 6 (1997) 446-54.
https://www.emf-portal.org/en/article/1257
Lai, H., and Singh, N.P. (1997c) "Melatonin and N-tert-butyl-a-phenylnitrone Block 60 Hz magnetic field-induced DNA single- and double-strands Breaks in Rat Brain Cells." Journal of Pineal Research 22:152-162.
Magnetic-Field-Induced
DNA Strand Breaks in Brain Cells of the Rat
HENRY
LAI & NARENDRA P SINGH / Environmental Health Perspectives v.112, n.6,
1may04
[All
figures below references]
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1241963/pdf/ehp0112-000687.pdf
http://www.voltimum.se/files/it/others/8/200403115810campi_elettromagnetici.pdf
Lai, H. "Non-Ionizing Electromagnetic Fields and Spatial Learning and Memory Functions." In Bersani (1999) 101-103.
http://link.springer.com/chapter/10.1007%2F978-1-4615-4867-6_22
SECTION 9
Neurological Effects of Non-Ionizing
Electromagnetic Fields
2014 Supplement
Henry
Lai
Lai, H. and Singh, N.P., 1996a: "Reply to "Comment on 'Acute low-intensity microwave exposure increases DNA single-strand breaks in rat brain cells' ". Bioelectromagnetics 17: 166.
Lai, H. and N.P. Singh (1996). "Single and double strand DNA breaks in rat brain cells after acute exposure to radiofrequency electromagnetic radiation." J Radiation Biology 69: 513-521.
More citations:
https://scholar.google.com/scholar?oe=utf-8&um=1&ie=UTF-8&lr&q=related:BJ62j0X6q_9dFM:scholar.google.com/
Article
May 2016 · Cell biochemistry and biophysics
Handan Kayhan· Meric
Arda Esmekaya· Atiye Seda Yar
SaglamNesrin
Seyhan
PICTURES AND VIDEOS
Can Cell Phones Damage DNA and Cause
Cancer? Dr. Henry Lai
Uploaded on Feb 26, 2012
“http://www.emrsafety.net
WiFi in Schools
![3469053_orig[1]](Lai%20Henry_files/image005.jpg)
http://www.wifiinschools.com/index.html
home-table1
This image is from:
http://greenswan.org/
WiFi in Schools
http://www.wifiinschools.com/index.html
3469053_orig[1].jpg
Dr. Henry Lai, from the University of Washington, gives a talk on the genetic effects of cell phones and Radiofrequency radiation.”
https://www.youtube.com/watch?v=JrBjQJhHfzkv
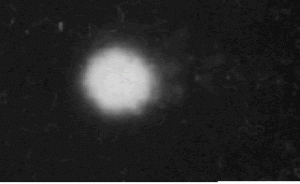
Lai, Henry. "Fig. 1. Unexposed control, The bundle is simply DNA."
Dna_fu1-300x184.gif
https://www.rfsafe.com/wp-content/uploads/2013/09/dna_fu1.gif
https://www.rfsafe.com/fact-dna-damage-safe-cell-phone-radiation-levels/
http://kundaliniandcelltowers.com/Cell%20Phone%20Radiation%20Danger%20Of%20Cancer%20DNA%20Damage.pdf
DNA Damage at Below Safe
Cell Phone Radiation Levels
l
https://preventcelltowersatmaplehillpark.files.wordpress.com/2015/05/cell-tower-presentation-2.pdf
Lai, Henry. "Fig. 2. X-ray calibration: After 6.4 rads. DNA strand breaks are evident.."
Previous link no longer valid.
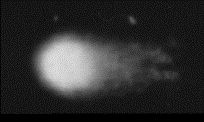
Lai, Henry. "Fig. 3. X-ray calibration: After 25.6 rads. DNA strand breaks are now very obvious.." https://www.rfsafe.com/fact-dna-damage-safe-cell-phone-radiation-levels/
 https://www.rfsafe.com/wp-content/uploads/2013/09/caldna.gif
https://www.rfsafe.com/wp-content/uploads/2013/09/caldna.gif
caldna.gif
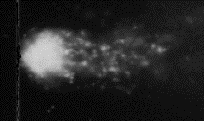
Lai, Henry. "Fig. 4 Assay showing effect of 2 hrs of microwave exposure (2.45GHz) at a SAR (absorption) level of 0.6 W/kg [about cellphone handset levels]. DNA strand breaks are also obvious."
dnamw.gif
https://www.rfsafe.com/fact-dna-damage-safe-cell-phone-radiation-levels/
https://www.rfsafe.com/wp-content/uploads/2013/09/dnamw.gif
Comet Assay
, in Encyclopedia
of Toxicology (Third Edition), 2014.
http://www.sciencedirect.com/topics/page/Comet_assay
References
1)
Resolution of Denial of Conditional Use ermit
(CUP) # PL2014-518 Cell Site At
Maple Hill Park, Diamond Bar, CA.
2)
Bio-initiative 2012, A Rationale for iologically
Based Exposure Standard for Low Intensity Electromagnetic Radiation
3)
Dr. Ronald Herberman,
Director of the University of Pittsburgh Cancer Institute (UPCI) and the UPMC
Cancer Center.
He is also Associate Vice Chancellor for cancer research within the
School of Medicine, Department of Health Sciences
.
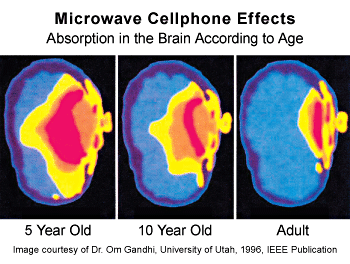
This is a modification of the iconic
illustration made in 1996 by Om Gandhi, Professor and Chairman of Department of
Electrical Engineering at the University of Utah, Salt Lake City. Dr. Herberman worked with Gandhi to turn the illustration into
a three-dimensional model that estimates the absorption of electromagnetic
radiation.
4)
Fact: DNA damage at below safe cell
phone radiation levels -
http://www.rfsafe.com/fact-dna-damage-safe-cell-phone-radiation-levels/
https://preventcelltowersatmaplehillpark.files.wordpress.com/2015/05/cell-tower-presentation-2.pdf
DNA Damage at Below Safe Cell Phone
Radiation Levels l
In a ground breaking series of
experiments between 1994–1998, Dr. Henry Lai and Dr. NP Singh demonstrated
convincingly that moderate levels of microwave (2.45 GHz) radiation (below that
of cell phone radiation levels) for exposure of 2 hours, could increase the
frequency of single-strand DNA break in brain cells of live animals.
4l
Accumulative and long term DNA
disruption may lead to cancer cell development
Fig.1
Unexposed control. The bundle is simply DNA
Fig.2 X-ray calibration: After 25.6 rads. DNA
strand breaks are now very obvious
p. 7 n.4
dnamw.gif
https://www.rfsafe.com/wp-content/uploads/2013/09/dnamw.gif
Fig.4 Assay showing effect of 2 hrs
of microwave exposure (2.45GHz) at a SAR (absorption) level of 0.6 W/kg [about
cell phone radiation levels] DNA strand breaks re also obvious.
These images result from fluorescent
molecules attached to the end of each DNA strand at a break point, and so are
best seen in the negative.
Figure 4 was captured by Dr Lai and
Singh, and it shows the results of a comet assay at power densities about
one-fifth those previously thought to cause adverse biological effects. These
exposures were only for a short time, and they used radio power-densities well
below those said to be ‘ionizing’ (having the power to break chemical/material
bonds).
https://www.rfsafe.com/fact-dna-damage-safe-cell-phone-radiation-levels/
Singh NP, Lai H. (1998) 60 Hz
magnetic field exposure induces DNA crosslinks in rat
brain
cells. Mutat Res 400:313-320.
from Public - City Clerk Internet Site - City of Los Angeles
May
16, 2005
p.1
To :
His Excellency Ban Ki - moon, Secretary - General of the United Nations
Honorable Dr. Margaret Chan,
Director-General of the World Health Organization
Honorable Achim
Steiner, Executive Director
Of the U.N. Environmental Programme
U.N. Member Nations
International Appeal: Scientists
call for Protection from Non-ionizing Electromagnetic Field Exposure
http://clkrep.lacity.org/onlinedocs/2013/13-0953_pc_05-05-16c.pdf
13
Lai H, Singh NP. 1997. Acute
exposure to a 60 Hz magnetic field increases DNA strand
breaks in rat brain cells. Bioelectromagnetics
18:156-65.
https://ecfsapi.fcc.gov/file/10811208526661/ELF%20-%20Lai,%20Singh,%201997.pdf
|
“The Comet Assay, also called single cell gel electrophoresis (SCGE), is a sensitive and rapid technique for quantifying and analyzing DNA damage in individual cells. As such, this is one of the techniques used in the area of cancer research for the evaluation of genotoxicity and effectiveness of chemoprevention. Swedish researchers Östling & Johansson developed this technique in 1984.1 Singh, et al., later modified this technique, in 1988, as the Alkaline Comet Assay.2 The resulting image that is obtained resembles a "comet" with a distinct head and tail. The head is composed of intact DNA, while the tail consists of damaged (single-strand or double-strand breaks) or broken pieces of DNA. While most of the applications of the Comet Assay have been to study animal eukaryotes, there have been reports of successful application in the study of plant cells. “Individual cells are embedded in a thin agarose gel on a microscope slide. All cellular proteins are then removed from the cells by lysing. The DNA is allowed to unwind under alkaline/neutral conditions. Following the unwinding, the DNA undergoes electrophoresis, allowing the broken DNA fragments or damaged DNA to migrate away from the nucleus. After staining with a DNA-specific fluorescent dye such as ethidium bromide or propidium iodide, the gel is read for amount of fluorescence in head and tail and length of tail. The extent of DNA liberated from the head of the comet is directly proportional to the amount of DNA damage. “The Comet Assay can be used to detect DNA damage caused by double strand breaks, single strand breaks, alkali labile sites, oxidative base damage, and DNA cross-linking with DNA or protein. The Comet Assay is also used to monitor DNA repair by living cells.3
Cited References
Review Articles and General References for Comet Assays
|
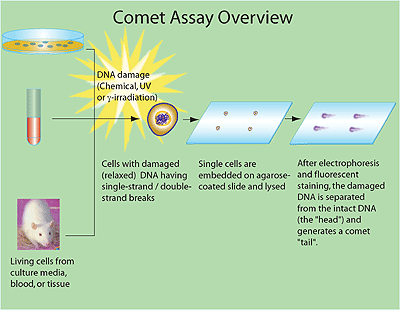
Comet-assay.gif
[Fig. 3]
https://www.rfsafe.com/wp-content/uploads/2013/09/caldna.gif
[Fig.1]
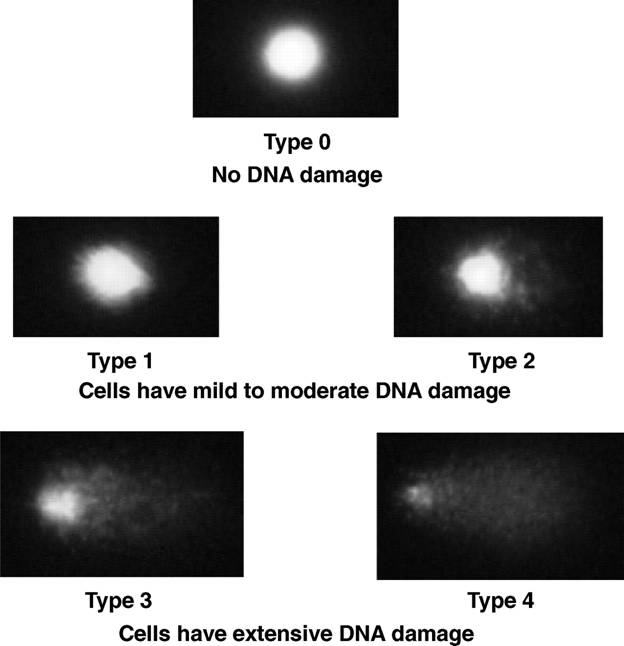
F1.large.jpg
http://cebp.aacrjournals.org/content/cebp/16/9/1906/F1.large.jpg
Figure1.
Measurement of DNA damage in PBLs
and prostate cells from elderly dogs.
The extent of DNA damage in PBLs and prostate cells was measured by single cell
gel electrophoresis (alkaline Comet assay) as described by Singh (13). Under
the assay conditions used in this experiment, comet tails reflect the electrophoretic migration of DNA fragments that result from
strand breaks, alkali-labile sites, crosslinks, or
base-excision repair sites (13). The extent of DNA damage was visually
scored in 100 randomly selected cells from each sample (50 cells
from several different fields from each of two replicate slides) by one
examiner who was blinded to the treatment group. SYBR Green 1 – stained nucleoids were examined at 200
magnification with an epifluorescent
microscope. Each cell was visually scored on a 0 to 4 scale using a method
described by Collins (42) as follows: no damage (type 0); mild to moderate
(types 1 and 2), and extensive DNA damage (types 3 and 4). The extent of DNA
damage within PBLs or prostate cells was expressed as the percentage of cells
with extensive DNA damage (sum of cells that displayed type 3 or type 4 DNA damage).
PBLs isolated from the whole blood of each dog (12) were assayed fresh without
cryopreservation. Cytospin preparations confirmed
that more than 90% of cells in this enriched cell population were lymphocytes;
mean percent viability
(trypan blue exclusion) was 90%. The sensitivity of
PBLs to oxidative stress was determined by measuring DNA damage
before and after exvivo challenge of
PBLs with 25 Amol/L hydrogen peroxide (5 min, 4j
C). To
assess endogenous DNA
damage in prostate cells, the prostate was collected from each dog
at necropsy, and 50 to 80 mg of prostate tissue was placed
in 1 mL of cold HBSS containing 20 mmol/L EDTA and 10% DMSO (43). Tissue was then minced with
fine scissors, and 50 AL of the resulting cell suspension was mixed with 1 mL of RPMI 1640 containing 10% fetal bovine serum for
subsequent
electrophoresis. Cytospin preparations indicated
that >90% of cells had epithelial cell morphology; mean percentage cell
viability estimated by trypan blue
exclusion was 80%. Histopathologic
evaluation of formalin-fixed, step-sectioned prostate tissue sections revealed
no foci of carcinoma in any of the dogs in this study population.”
This figure is from:
Noninvasive Prediction of Prostatic DNA Damage by
Oxidative Stress Challenge of Peripheral Blood Lymphocytes
David J.WatersShurenShenHuipingXuSeema S.KengeriDawn M.CooleyEmily C.ChiangYuChenDeborahSchlittlerCarolOtehamGerald F.CombsJr.Lawrence T.GlickmanJ. StevenMorrisDavid G.Bostwick
DOI:
http://cebp.aacrjournals.org/content/cebp/16/9/1906.full.pdf